Enthalpy Change of Solution
The enthalpy of vaporization is often. According to the definition of enthalpy of neutralization chem libretexts the standard enthalpy change of neutralization is the enthalpy change when solutions of an acid and an alkali react together under standard conditions to produce 1 mole of water.
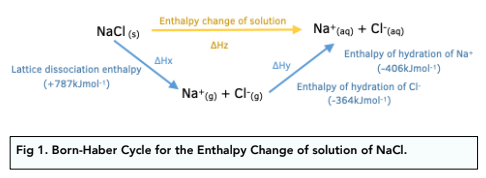
Thermodynamic Calculating Enthalpy Changes Of Solution A Level Chemistry Study Mind
By putting the values of pressure internal energy and change in volume we can calculate change in enthalpy of the system as follows.

. Using reaction scheme Determine change in enthalpy of the chemical reaction as. On the other hand the formation of a chemical bond is almost always an endothermic process. CaCl 2 s aq CaCl.
The isentropic efficiency of turbine can then be written as. It is the amount of heat absorbed or liberated when a substance is dissolved in a solvent to form an infinitely dilute solution. The atomization of dihydrogen molecule.
As we know that the enthalpy change formula is given as. Enthalpy Change of Solution. Enthalpy change of solution may be positive or negative and is denoted by H sol.
H 2 a H 0 4350 kJ mol-1. ΔH ΔQ p ΔV. Thus the enthalpy change associated with the breaking of a chemical bond is always positive ΔH 0.
ηT h 2a - h 1h 2s - h 1 where h 1 enthalpy at the inlet. In such cases the enthalpy change will have a negative value ΔH 0. Enthalpy of the solution Δ sol H 0 is the enthalpy change when one mole of a substance is completely dissolved in a solvent.
For diatomic molecules the enthalpy of atomization is equal to the enthalpy of bond dissociation. The enthalpy of vaporization symbol H vap also known as the latent heat of vaporization or heat of evaporation is the amount of energy that must be added to a liquid substance to transform a quantity of that substance into a gasThe enthalpy of vaporization is a function of the pressure at which that transformation takes place. Usually the kinetic and potential energies associated with a process through a turbine is negligible compared with the enthalpy change of the process.
The value of enthalpy change is positive because this reaction is endothermic. Energy is required to break or make one mole of particular. In this case the energy balance of the turbine is reduced to.
I highlighted 1 mole of water because thats what I used to solve the problem.

15 1 Enthalpy Change Of Solution And Hydration Hl Youtube

Thermodynamics Does The Enthalpy Of Solution Formula Le Hyd Change Depending On The Question Chemistry Stack Exchange


No comments for "Enthalpy Change of Solution"
Post a Comment